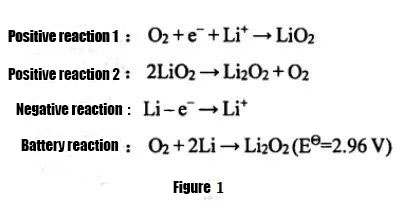01 Cov roj teeb lithium-air thiab lithium-sulfur roj teeb yog dab tsi?
① Li-air roj teeb
Lub roj teeb lithium-air siv oxygen raws li qhov zoo electrode reactant thiab hlau lithium raws li qhov tsis zoo electrode.Nws muaj lub zog theoretical siab ceev (3500wh / kg), thiab nws qhov tseeb lub zog ceev tuaj yeem ncav cuag 500-1000wh / kg, uas yog ntau dua li cov roj teeb lithium-ion.Cov roj teeb lithium-air yog tsim los ntawm cov electrodes zoo, electrolytes thiab tsis zoo electrodes.Hauv cov roj teeb uas tsis muaj dej, cov pa ntshiab yog tam sim no siv los ua cov pa roj, yog li cov roj teeb lithium-air kuj tseem hu ua lithium-oxygen roj teeb.
Xyoo 1996, Abraham et al.ua tiav thawj zaug uas tsis yog-aqueous lithium-air roj teeb hauv chav kuaj.Tom qab ntawd cov kws tshawb fawb tau pib ua tib zoo mloog rau cov tshuaj tiv thaiv hauv electrochemical thiab cov txheej txheem ntawm cov roj teeb lithium-air tsis muaj dej;xyoo 2002, Read et al.pom tias qhov kev ua tau zoo ntawm electrochemical ntawm lithium-air roj teeb nyob ntawm cov khoom siv electrolyte hnyav thiab cov khoom cua cathode;Xyoo 2006, Ogasawara et al.siv Mass spectrometer, nws tau ua pov thawj thawj zaug uas Li2O2 tau oxidized thiab oxygen tau tso tawm thaum lub sij hawm them nyiaj, uas tau lees paub cov electrochemical reversibility ntawm Li2O2.Yog li ntawd, cov roj teeb lithium-air tau txais kev mloog ntau thiab kev loj hlob sai.
② Lithium-sulfur roj teeb
Lithium-sulfur roj teeb yog lub roj teeb thib ob raws li cov tshuaj tiv thaiv rov qab ntawm cov muaj peev xwm tshwj xeeb sulfur (1675mAh / g) thiab lithium hlau (3860mAh / g), nrog qhov nruab nrab tawm hluav taws xob ntawm li 2.15V.Nws qhov theoretical zog ceev tuaj yeem ncav cuag 2600wh / kg.Nws cov ntaub ntawv raw muaj qhov zoo ntawm tus nqi qis thiab kev phooj ywg ib puag ncig, yog li nws muaj peev xwm loj hlob zoo.Kev tsim cov roj teeb lithium-sulfur tuaj yeem taug qab mus rau xyoo 1960, thaum Herbert thiab Ulam tau thov rau roj teeb patent.Cov qauv ntawm cov roj teeb lithium-sulfur no siv lithium lossis lithium alloy ua cov khoom siv tsis zoo electrode, sulfur li cov khoom siv electrode zoo thiab muaj li ntawm aliphatic saturated amines.ntawm electrolyte.Ob peb xyoos tom qab ntawd, cov roj teeb lithium-sulfur tau txhim kho los ntawm kev qhia cov kuab tshuaj organic xws li PC, DMSO, thiab DMF, thiab 2.35-2.5V roj teeb tau txais.Thaum xyoo 1980s, ethers tau ua pov thawj tias muaj txiaj ntsig zoo hauv cov roj teeb lithium-sulfur.Hauv cov kev tshawb fawb tom ntej, kev tshawb pom ntawm ether-based electrolytes, kev siv LiNO3 ua cov khoom siv electrolyte, thiab cov lus pom zoo ntawm carbon / sulfur composite electrodes tau qhib qhov kev tshawb fawb boom ntawm lithium-sulfur roj teeb.
02 Lub hauv paus ntsiab lus ua haujlwm ntawm lithium-air roj teeb thiab lithium-sulfur roj teeb
① Li-air roj teeb
Raws li lub xeev sib txawv ntawm cov electrolyte siv, lithium-air roj teeb yuav muab faib ua aqueous systems, organic systems, water-organic hybrid systems, and all-solid-state lithium-air batteries.Ntawm lawv, vim qhov muaj peev xwm tshwj xeeb ntawm lithium-air roj teeb uas siv dej-raws li electrolytes, muaj teeb meem hauv kev tiv thaiv lithium hlau, thiab tsis zoo rov qab los ntawm lub cev, cov roj ntsha lithium-air tsis muaj dej thiab tag nrho-lub xeev lithium-air. cov roj teeb tau siv dav dua tam sim no.Kev tshawb fawb.Cov roj teeb uas tsis yog-aqueous lithium-air yog thawj zaug tau thov los ntawm Abraham thiab Z.Jiang hauv xyoo 1996. Qhov sib npaug ntawm cov tshuaj tiv thaiv tawm tau pom hauv daim duab 1. Cov tshuaj tiv thaiv kev them nyiaj yog qhov sib txawv.Cov electrolyte tsuas yog siv cov organic electrolyte los yog cov khoom electrolyte, thiab cov khoom tso tawm yog tsuas yog Li2O2, cov khoom yog insoluble nyob rau hauv cov electrolyte, thiab nws yog ib qho yooj yim mus tsub zuj zuj ntawm cov huab cua zoo electrode, cuam tshuam lub peev xwm ntawm lub lithium-air roj teeb.
Lithium-air roj teeb muaj qhov zoo ntawm ultra-siab zog ceev, ib puag ncig tus phooj ywg, thiab tus nqi qis, tab sis lawv cov kev tshawb fawb tseem nyob rau hauv nws cov me nyuam mos, thiab tseem muaj ntau yam teeb meem yuav tsum tau daws, xws li catalysis ntawm oxygen txo cov tshuaj tiv thaiv, cov oxygen permeability thiab hydrophobicity ntawm huab cua electrodes, thiab deactivation ntawm huab cua electrodes thiab lwm yam.
② Lithium-sulfur roj teeb
Cov roj teeb lithium-sulfur tsuas yog siv cov tshuaj sulfur lossis sulfur-based compounds raws li cov khoom siv hluav taws xob zoo ntawm lub roj teeb, thiab cov roj ntsha lithium feem ntau yog siv rau qhov tsis zoo electrode.Thaum lub sij hawm tso tawm cov txheej txheem, cov hlau lithium nyob ntawm qhov tsis zoo electrode yog oxidized kom poob ib qho hluav taws xob thiab tsim cov lithium ions;Tom qab ntawd cov electrons raug xa mus rau qhov zoo electrode los ntawm sab nraud Circuit Court, thiab cov lithium ions generated kuj raug xa mus rau qhov zoo electrode los ntawm cov electrolyte los teb nrog sulfur los ua polysulfide.Lithium (LiPSs), thiab tom qab ntawd rov ua dua los tsim cov lithium sulfide kom ua tiav cov txheej txheem tawm.Thaum lub sij hawm them nyiaj, lithium ions hauv LiPSs rov qab mus rau qhov tsis zoo electrode los ntawm electrolyte, thaum cov hluav taws xob rov qab mus rau qhov tsis zoo electrode los ntawm ib qho hluav taws xob sab nraud los tsim cov lithium hlau nrog lithium ions, thiab LiPSs raug txo kom sulfur ntawm qhov zoo electrode kom tiav. kev them nqi.
Cov txheej txheem tso tawm ntawm lithium-sulfur roj teeb feem ntau yog ntau kauj ruam, ntau lub tshuab hluav taws xob, ntau theem complex electrochemical cov tshuaj tiv thaiv ntawm cov sulfur cathode, thiab LiPSs nrog cov saw ntev sib txawv tau hloov mus rau ib leeg thaum lub sijhawm them nqi.Thaum lub sijhawm tso tawm, cov tshuaj tiv thaiv uas yuav tshwm sim ntawm qhov zoo electrode yog qhia hauv daim duab 2, thiab cov tshuaj tiv thaiv ntawm qhov tsis zoo electrode yog qhia hauv daim duab 3.
Qhov zoo ntawm cov roj teeb lithium-sulfur yog qhov pom tseeb heev, xws li lub peev xwm theoretical siab heev;tsis muaj cov pa oxygen hauv cov khoom, thiab cov tshuaj tiv thaiv oxygen evolution yuav tsis tshwm sim, yog li kev nyab xeeb kev ua tau zoo;sulfur cov peev txheej muaj ntau thiab cov ntsiab lus sulfur yog pheej yig;nws yog ib puag ncig tus phooj ywg thiab muaj toxicity tsawg.Txawm li cas los xij, cov roj teeb lithium-sulfur kuj muaj qee qhov teeb meem nyuaj, xws li lithium polysulfide shuttle effect;rwb thaiv tsev ntawm cov hlau sulfur thiab nws cov khoom tso tawm;qhov teeb meem ntawm kev hloov pauv loj;qhov tsis ruaj tsis khov SEI thiab teeb meem kev nyab xeeb tshwm sim los ntawm lithium anodes;tus kheej tawm phenomenon, thiab lwm yam.
Raws li lub cim tshiab ntawm cov roj teeb nruab nrab, cov roj teeb lithium-air thiab lithium-sulfur roj teeb muaj qhov muaj peev xwm theoretical siab heev, thiab tau nyiam cov neeg tshawb nrhiav thiab cov lag luam roj teeb thib ob.Tam sim no, ob lub roj teeb no tseem tab tom ntsib ntau yam teeb meem kev tshawb fawb thiab thev naus laus zis.Lawv nyob rau theem pib tshawb fawb ntawm kev txhim kho roj teeb.Ntxiv rau qhov tshwj xeeb muaj peev xwm thiab kev ruaj ntseg ntawm cov khoom siv roj teeb cathode xav tau kev txhim kho ntxiv, cov teeb meem tseem ceeb xws li roj teeb kev nyab xeeb kuj yuav tsum tau kho sai.Nyob rau yav tom ntej, ob hom roj teeb tshiab no tseem xav tau kev txhim kho txuas ntxiv txhawm rau tshem tawm lawv qhov tsis xws luag txhawm rau qhib qhov kev thov dav dav.
Post lub sij hawm: Apr-07-2023